What is the NuvaRing® and how does it differ from other birth control methods?

NuvaRing®, (pictured on the right) is a soft, flexible ring that is approximately two inches in diameter. The ring is worn in the vagina for three weeks. It is then removed for one week. The menstrual period will usually occur during this week.
NuvaRing® contains a combination of estrogen and progestin, hormones similar to the hormones used in most birth control pills and in the patch. The primary difference is in the method by which the medication is delivered into the body. The hormones contained within the ring are absorbed continuously, directly into the blood stream through the vaginal wall.
How does the ring work?
The estrogen and progestin hormones are similar to the natural hormones produced by the ovaries. The progestin component is absorbed in small amounts and prevents the ovary from releasing an egg (ovulation). The uterine lining also becomes thinner than usual, which would inhibit implantation of a fertilized egg. In addition, the cervical mucus also becomes thicker and this helps prevent sperm from entering the uterus. When properly placed, the ring is held in place by the vaginal muscle structure, even during exercise or intercourse. The NuvaRing® cannot “get lost” in the vagina. The vagina is flexible, but no longer than the palm of your hand. The cervix forms the back wall of the vagina so the ring remains in the vagina.
How effective is NuvaRing®?
According to studies provided to the Food and Drug Administration (FDA) during the approval process, effectiveness ratings are listed at 99% when used according to label instructions. This is comparable to the patch and better than the Pill’s effectiveness ratings.
What are the benefits of using NuvaRing®?
- Medication is absorbed directly into the blood stream through the vaginal wall membrane providing a consistent level of medication in the blood, which improves effectiveness and helps limit side effects. Oral contraceptives take time to be absorbed into the blood stream and this causes peaks and valleys in the hormone blood levels.
- Once monthly insertion eliminates the need to think about birth control on a daily or weekly basis; usually neither partner is aware of its presence during intercourse.
- Very discreet and private; no one can see it or know that you are wearing it.
- Easily reversible – ovulation returns quickly when use of the ring is discontinued; however, women who have irregular menstrual cycles will probably return to the irregular cycle.
- Provides a regular menstrual period, but menses are usually lighter and may be shorter than a normal period.
- Thickened cervical mucus helps decrease the risk of pelvic inflammatory infections.
- Reduces the chances of ovarian cysts and tubal pregnancy.
- Vomiting and diarrhea should not interfere with the effectiveness of the ring.
Are there other things that should be considered?
Insertion and removal require that a woman insert her finger inside the vagina; therefore she must be comfortable touching her genitalia and vagina.
- NuvaRing® does not offer any protection against transmission of sexually transmitted infections (STDs), such as chlamydia, gonorrhea, syphilis, hepatitis or HIV. Condoms may be used with the ring to reduce the risk of STDs.
- Always inform your health care provider that you are using the ring. It is a medication just like any others that you are taking.
- Disposal is easy – Keep the original foil package and when the ring is removed just place it back in the foil pack, seal and throw away.
- NuvaRing® is nonabsorbent, odorless, and contains no latex.
- Do not breast feed while using NuvaRing®.
- If for any reason, the ring is removed or slips out, it may be rinsed in cool water and reinserted. If it is out for three hours or more, there will be reduced contraceptive protection and an alternative method of birth control must be used until the ring has been in place for seven consecutive days. Emergency contraception is available if it is out three hours or longer.
- Unopened NuvaRing® packs will expire in four months unless stored in the refrigerator. (Refer to the package insert for additional information or talk with the pharmacist.)
- If unopened NuvaRing® packs are stored in temperatures greater than 86° the effectiveness may be lessened.
- It is safe to use other vaginal medications/lubricants with the NuvaRing®.
What about cancer and the NuvaRing®?
There is little information available specifically about the ring and cancer. However, since the ring and birth control pills utilize the same types of hormones, it is expected to have similar effects. That information is included for your review.
Since 1960, when birth control pills first became available, important information has been learned about pills and cancer. Pills reduce the risk of ovarian cancer – three years of use reduces the risk of ovarian cancer by 40%; ten years reduces the risk by 80%. Pills reduce the risk for endometrial (uterine lining) cancer. Most studies suggest that pills neither reduce nor increase the risk for breast cancer. Research continues to evaluate this. Women are encouraged to do monthly self-breast exams and report any changes or problems to their health care provider. Some studies indicate an increased incidence of cervical cancer. However, this may be more related to factors such as numbers of sexual partners, STD exposure, etc., than to birth control pill use. Annual pap smears provide the best screening for cervical cancer.
What about drug interactions?
Contraceptive effectiveness may be reduced when hormonal contraceptives are administered at the same time other drugs are being taken. Break through bleeding or unintended pregnancy can result. Examples of drugs that potentially interfere with contraceptive hormones include antifungals, seizure medications and, potentially, antibiotics. For additional information please refer to the handout Pill Interactions with Other Drugs.
Are there risks associated with use of NuvaRing®?
Health risks related to the contraceptive ring are low when compared to the risks of pregnancy and childbirth and are the same as with the birth control pill and the patch. The risks are usually associated with the estrogen component in the ring. In a small number of women estrogen affects the way the body forms blood clots. This may cause blood clots to form in the legs, lungs, brain or other vital organs causing serious health problems.
Cigarette smoking increases the risk of cardiovascular complications and is greater when a woman smokes more than 15 cigarettes daily and increases significantly when a she is also over 35 years of age. Women are advised to quit smoking if they use hormonal contraception in any form.
Other factors increasing the risk of blood clots are high cholesterol, diabetes, high blood pressure, obesity, and migraine headaches with aura or neurological symptoms.
If you experience any of the following symptoms, you should seek medical care right away:
A – Abdominal pain (severe)
C – Chest pain, shortness of breath, coughing up blood
H – Headache (severe), numbness or weakness in arms or legs
E – Eye problems (vision loss, blurring, flashing lights)
S – Severe leg pain in calf or thigh
What are the side effects?
The estrogen component is responsible for many of the side effects that occur with all of the combined hormonal methods of birth control (ring, patch or pill). The ring has less estrogen than either the pill or the patch; therefore nausea, headaches and breast tenderness may occur less often or be less severe.
The most common side effect is an increased amount of normal vaginal discharge. Occasionally women report an increased number of vaginal infections and irritation. Clinical studies show women using the ring experience an increased amount of lactobacillus in the vagina. Lactobacillus is a normal bacteria present in the vagina that actually helps decrease the incidence of yeast infections.
Headaches may occur. These should be reported to your health care provider right away if the headache or accompanying symptoms (light sensitivity, nausea, vomiting, etc.) is more severe than you have experienced before, do not respond to over-the-counter medications or last more than one month.
Mild nausea may occur with initial use, but usually will resolve within a few days. Rarely, vomiting may occur. If nausea lasts throughout the month or occurs persistently at the beginning of every ring cycle, report it to your health care provider. Vitamin B-6 (50 mg. 1-2 tablets daily) may help reduce nausea.
Weight gain may occur, but is usually limited to a few pounds.
Moodiness may occur within the first 1-2 months of use, but will usually diminish with continued use. Vitamin B-6 (50 mg. 1-2 tablets daily) may help decrease moodiness as well as relieve nausea. Notify your provider right away if severe depression symptoms occur.
Occasionally spotting and mid-cycle bleeding may occur while the ring is in the vagina. The amount of bleeding is usually minimal and will normally be reduced as you continue use, but may occur from time to time for no apparent reason. This is a normal occurrence and does not indicate that it is not working as a contraceptive.
Reactions to any method of birth control will vary from person to person. For some the reaction will be beneficial, but others may find them detrimental. For instance, acne may improve for some, but worsen for others; cramps usually improve, but occasionally a woman will experience increased cramps instead.
Is it safe to use vaginal medications or lubricants when the ring is in place?
Yes, you may use vaginal medications or water-based lubricants while the NuvaRing® is in place in the vagina. There will be no decrease in the contraceptive benefit. NuvaRing® is a medication and you should always advise all health care providers that you are using NuvaRing® for contraception.
When do I start using NuvaRing®?
If you are starting NuvaRing® and HAVE NOT been using any other hormonal method of birth control (i.e. birth control pills or the patch): insert the ring between day one and five of your menses, even if you have not finished bleeding. Count the first day of your period as day one. You should use a condom during each act of intercourse for the first seven days during the first ring cycle.
– OR –
Quick Start: Insert the NuvaRing® the day of your appointment with your provider, regardless of where you are in your menstrual cycle. This allows you to start using NuvaRing® immediately, without waiting until you get your period. If you use the Quick Start method, it is necessary to use condoms or another back-up method of birth control for seven days. Women concerned about a possible pregnancy before starting the NuvaRing® should wait until they get their period and insert the ring between day one and day five of menses. If using the Quick Start method, your period may be delayed until your ring free week. If you do not get a period during your ring free week, take a urine pregnancy test. You may experience break through bleeding with Quick Start; leave the NuvaRing® in place, even if unscheduled bleeding occurs.
If you are switching from birth control pills to the NuvaRing®: Insert the ring at any time during the seven days immediately following your last active birth control pill.It may be easiest to insert the ring on the day that you would have normally started a new pill pack. There should never be more than seven days between the last active pill and insertion of the ring. The ring may be inserted even if there is still some bleeding. No back up birth control is needed.
If you are switching from a progestin-only pill (POP): Insert the NuvaRing® on any day of the month. Do not skip any days between your last pill and the first day of NuvaRing® use. Use a back-up method of birth control (condoms) for seven days.
If you are switching from the patch to NuvaRing®: Insert the ring at any time during the seven days immediately following the removal of Patch #3. There should be no more than seven days between removing the patch and inserting the ring. The ring may be inserted even if there is still some bleeding. No back up birth control is needed.
If switching from DepoProvera® to NuvaRing®: Insert NuvaRing® on the day your next injection is due. (12 weeks from previous injection plus or minus a week) Use a back up method of birth control (condoms) for seven days.
If starting NuvaRing® after a first trimester abortion: Insert NuvaRing® within the first five days after the abortion. No back up birth control is needed. If not started within the first five days, wait until the next menstrual period starts and insert within the first five days of bleeding. During the first cycle, use a back up method of birth control for seven days.
When do I remove the NuvaRing®?
Remove the ring three weeks after insertion, at approximately the same time of day, if possible. Place it in the foil envelope in which it was originally packaged and throw away. Contraceptive protection continues throughout the ring-free week.
When do I insert a new ring?
Seven days after removing the old ring, insert a new one. It is recommended that you insert and remove it at the same time of day each month. For example if you insert the first ring at 7 a.m. Sunday morning, it should be removed at approximately 7 a.m. on Sunday three weeks later and a new ring inserted on the following Sunday at approximately the same time. The ring may be inserted sooner than this time if it is more convenient. It should not be inserted later than seven days.
You should expect your period during the ring-free week. Occasionally, due to the low dose of hormones used in the ring, you may experience a “missed” period (the absence of bleeding during the ring-free week). If this occurs, and the ring has been used properly, following all instructions, re-insert the ring as scheduled. For reassurance you may do a pregnancy test if you wish. If you miss two periods in a row, you should talk with your health care provider.
If the ring has been out of the vagina for less than or equal to 3 hours re-insert ring as soon as possible. Ring removal day stays the same. Contraceptive effective is not lost.
Insertion delayed greater than or equal to 24 hours – or- removal for greater that 3 hours:
- During Week 1 and removal is greater than 3 hours or unsure how long ring was removed:
- Reinsert ring as soon as possible.
- Ring removal day stays the same,
- Use condoms or abstain for 7 days.
- Consider emergency contraception if unprotected intercourse with in the previous 5 days.
- During Week 2 or 3 and removal <72 hours (<3 days):
- Re-insert ring as soon as possible.
- Ring removal day stays the same. Then start a new cycle with a new ring with no hormone free week.
- During Week 2 or 3 and removal greater that or equal to 72 hours (3 days)
- Re-insert ring as soon a possible.
- Ring removal day stays the same. Then start a new cycle with a new ring with no hormone free week.
- Use condoms or abstain for 7 days.
- Consider emergency contraception if repeated or prolonged omission of ring.
How do I insert and remove the NuvaRing®?
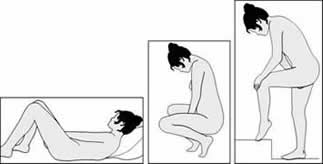
Positions for NuvaRing® Insertion
The NuvaRing® may be inserted while standing, squatting or lying down.
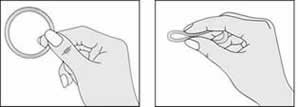
Hold NuvaRing® and Press Side Together
Using your thumb and index finger, press the sides of the ring together.
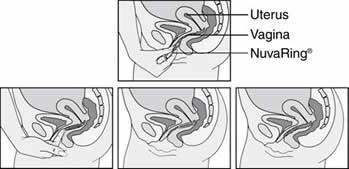
Inserting NuvaRing®
Gently push it deep into the vagina.
The exact position is not important. You should not be able to feel the ring when it is properly placed.
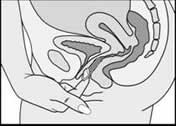
Removing NuvaRing®
To remove the ring, reach into the vagina using your index finger. Hook the tip of your finger around the ring and pull gently. The ring will naturally fold slightly as it is removed from the vagina.
Instructions for Missed Contraceptive Ring
What if I forget to remove the ring on time?
- If NuvaRing® remains in the vagina for 28 to 35 days: Insert a new ring with no hormone free week (week without ring in vagina). Keep it in until the scheduled ring removal day (21 days after insertion).
- If NuvaRing® remains in place longer than 35 days: Insert a new ring with no hormone free week. Leave the ring in for 21 days. Use condoms and spermicide or abstain for 7 days. Consider emergency contraception if unprotected intercourse occurred within previous 5 days. If you do not have a menstrual cycle in three weeks, when you remove the NuvaRing® you should do a home pregnancy test.
What if the ring slips out?
If the ring is not inserted deep into the vagina it may slip out when straining to have a bowel movement or removing a tampon. If this occurs, rinse it with cool water and reinsert it as soon as possible. If it is out for less than three hours, there is no loss of contraceptive protection. Ring removal day stays the same.
If the ring is lost, reinsert a new ring and use it following the original insertion/removal schedule. If the ring is out of place for more than three hours, you must use condoms as a back up birth control method for seven days. You may also wish to consider using Plan B Emergency Contraception.
If you are having problems with the ring slipping out of place frequently, talk with your health care provider.
References
- Missed Doses of Hormonal Contraceptive. Pharmacist’s Letter/Prescriber’s Letter 2009; 25(1):250120.
- UptoDate Online 18.1 Jan 2010.
- NuvaRing web site: http://www.nuvaring.com/
- Drawings © 2008 Organon USA Inc.








